دانلود نرم افزار Orbitab Viewer
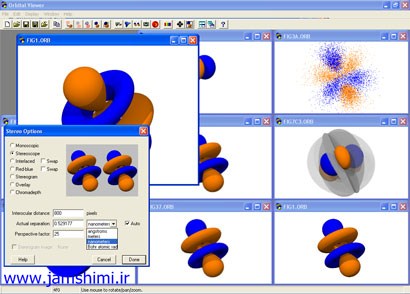
Orbitab Viewer یک نرم افزار بسیار قوی شیمی و در عین حال کاربرپسند و ساده برای نمایش انواع اوربیتال ها و انجام انواع محاسبات کوانتومی مربوط به آنها به همراه فایل های نمونه که در ادامه از جم شیمی دانلود می کنید.
The electron orbitals presented here represent a volume of space within which an electron would have a certain probability of being based on particular energy states and atoms. For example, in a simple lowest-energy state hydrogen atom, the electrons are most likely to be found within a sphere around the nucleus of an atom. In a higher energy state, the shapes become lobes and rings, due to the interaction of the quantum effects between the different atomic particles. In addition to technical merits, they make pretty pictures.
The shape of the orbital depends on many factors. The most important are the quantum numbers associated with the particular energy state. These are n, the principal quantum number, l, the orbital quantum number, and m, the angular momentum quantum number. The following table shows some of these shapes. Also available is the Grand Table, showing many, many more orbitals in six different organizations.